© Copyright
Department of Integrative Neuroscience,
Graduate School of Biomedical Sciences,
Nagasaki University
From anatomy to the mind, from physiology to the mind, and from a patient-first perspective in neuropathology, our department seeks to approach the workings of the basic neuroscience and address research questions of patients suffering from neurological disorders through these “three arrows.”
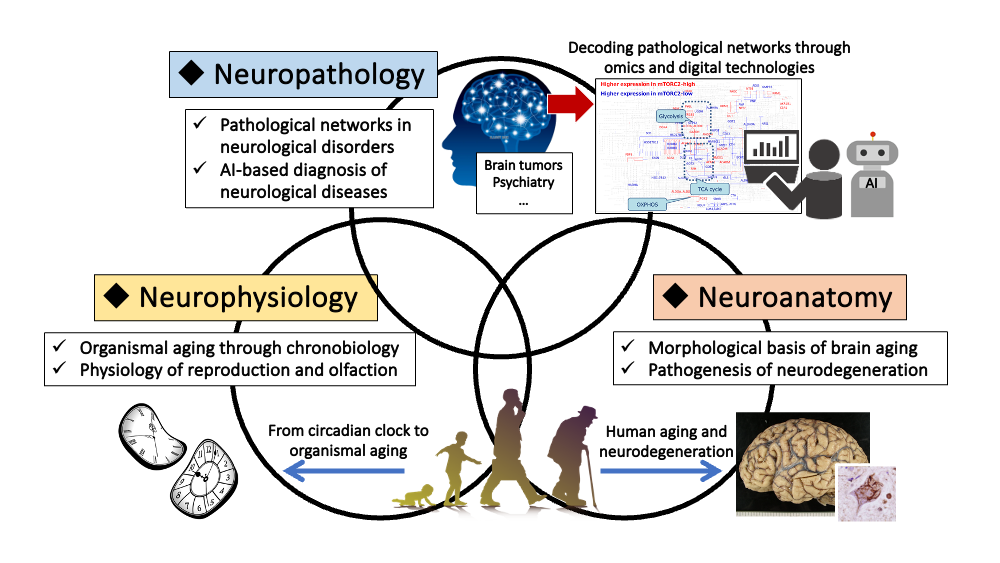
Brain tumor research represents an interdisciplinary field bridging neurodevelopment and tumor biology. Our group focuses on the theme of “cancer metabolism,” which underlies the diverse phenotypes that cancer cells can adopt. By integrated analyses of human brain tumor specimens and glioma models through genetic modification of glial progenitor cells (Masui et al. GLIA 2010), we were the first to demonstrate that the mTOR complex, a key regulator of intracellular metabolism, functions as a central driver of cancer metabolism (Masui et al. Cell Metab 2013). These metabolic alterations exert widespread effects on cancer cells, conferring resistance to molecular targeted therapies (Masui et al. PNAS 2015) and profoundly reshaping the epigenomic landscape, including histone modifications and DNA methylation (Masui et al. JBC 2019; Harachi et al. Mol Cancer Res 2020). Furthermore, we recently uncovered that metabolism-dependent epigenomic changes in malignant gliomas remodel neuron-glioma synaptic networks in the brain, thereby promoting tumor progression—an emerging frontier that unites brain tumor biology and neuroscience (Harachi et al. Acta Neuropath Commun 2024). Moving forward, we aim to decipher the pathological networks formed by neuron-glioma synapses, elucidate how synaptic connectomes contribute to neurological and psychiatric disorders, and pioneer next-generation diagnostic technologies by digitally decoding disease networks with AI.
We have long investigated the molecular mechanisms of chronobiology, particularly the circadian clock. Beyond regulating sleep–wake rhythms, the circadian system governs hormone secretion, metabolism, and immunity, making it indispensable for maintaining health. We discovered that the key metabolite NAD⁺ oscillates under circadian control and drives the 24-hour rhythmic activation of the clock-regulated protein Sirt1 (Nakahata et al. Cell 2008; Science 2009). This was the first molecular demonstration that circadian clocks and metabolism are mutually coupled. By integrating the concept of aging, we further showed that elevated NAD⁺ delays cellular senescence (Khaidizar et al. Genes Cells 2017), while progression of cellular senescence accelerates circadian clock aging (Ahmed et al. Aging 2019; Front Neurosci 2021). We elucidated molecular mechanisms underlying circadian clock aging (Ashimori et al. Front Neurosci 2021), leading to the novel concept of “aging of the clock.” Currently, through collaborative projects with industry, we are identifying natural compounds capable of reversing circadian clock aging and investigating their mechanisms of action (Kuatov et al. Nutrients 2025). Building upon these insights, we aim to develop innovative preventive and therapeutic strategies for age-related and neuropsychiatric disorders.
Our research has addressed the mechanisms of neural stem/progenitor cell regulation, neuronal function, and their relevance to neurological disease. We demonstrated that the nuclear receptor TLX, a regulator of neural stem cells, is essential for adult neurogenesis and memory enhancement (Murai et al. PNAS 2014) and that TLX-mediated miRNA regulation is linked to schizophrenia (Murai et al. Nat Commun 2016). We also uncovered that G proteins interact with ephrins to control neural progenitor cell fate during cortical development (Murai et al. Stem Cells 2010; Qiu et al. Cereb Cortex Commun 2020). Building upon these findings, we now integrate morphological analyses of human tissue samples to explore the mechanisms of psychiatric and neurodegenerative disorders. Among these, Lewy body diseases, including Parkinson’s disease and dementia with Lewy bodies, are characterized by intracellular protein aggregates known as Lewy bodies. Pathological α-synuclein aggregates, the principal component of Lewy bodies, are thought to propagate between cells during disease progression, driving neurodegeneration and expanding pathology. By dissecting the propagation pathways of α-synuclein aggregates, we aim to elucidate the mechanisms of disease progression and contribute to the development of strategies for early diagnosis and prevention of neurodegenerative disorders.
We investigate the regulation of reproductive function at both the peripheral and central levels. At the ovarian level, we have analyzed follicle development and steroidogenesis, clarifying the critical role of the local microenvironment in maintaining fertility (Tarumi et al. Fertil Steril 2012; PLoS One 2014). On the central side, we have focused on the hypothalamus and pituitary, accumulating insights into the neuroendocrine system. Based on this foundation, we are currently examining the role of clock genes in reproductive aging, with particular attention to ovary-brain interactions. In parallel, we are exploring how olfactory stimuli influence brain function and behavior via hormonal responses. For example, we reported that body odors from women at ovulatory phase alter testosterone and cortisol levels in men (Tarumi et al. PLoS One 2020). We further found that inhalation of natural essential oils enhances oxytocin secretion in postmenopausal women, and that the olfactory stimulus β-caryophyllene elevates testosterone levels in women (Tarumi et al. J Alt Complement Med 2020; Sex Med 2020). Since both olfactory and reproductive functions are rooted in hypothalamic-pituitary neuroendocrine regulation, these two lines of research converge within a common framework. Looking ahead, we aim to deepen integrative understanding of reproductive aging and olfactory stimuli, ultimately contributing to improvements in healthcare and quality of life.